|
New Drug Treatment for Alzheimer's Disease
A molecule designed by a Purdue University researcher could lead to the first drug treatment for Alzheimer's disease. Shown is an image of the X-ray crystal structure of the molecule bound to memapsin 2, the enzyme responsible for amyloid plaque deposits in the human brain. The bond disables the enzyme and prevents the plaques that cause Alzheimer's disease. (Credit: Ghosh laboratories) "There are many people suffering, and no effective treatment is available to them," said Arun Ghosh, the Purdue professor who designed the molecule. "There is an urgent need for a drug to treat this devastating disease, and the scientific community has been working on this problem for many years." The National Institute on Aging estimates that as many as 4.5 million Americans suffer from Alzheimer's disease, which leads to dementia by affecting parts of the brain that control thought, memory and language. The new molecule prevents the first step in a chain of events that leads to amyloid plaque formation in the brain. The material at various stages of plaque formation is made up of fibrous clumps of toxic proteins that cause the devastating symptoms of Alzheimer's disease, said Ghosh, who has a dual appointment in the chemistry and medicinal chemistry and molecular pharmacology departments. "Interdisciplinary research and the tools available today allowed us to build a molecule that is both highly potent and highly selective, meaning it does not affect other enzymes important to brain function," he said. Jordan Tang, head of the Protein Studies Research Program at the Oklahoma Medical Research Foundation, is one of the discoverers of the critical enzyme and target for intervention, Ghosh said.
"This is the most exciting target today for Alzheimer's disease intervention," said Tang, who holds the J.G. Puterbaugh Chair in Medical Research at the Oklahoma Medical Research Foundation. "These interactions happen at a very early stage in the disease, and if we could block them, we could prevent many of the harmful steps that follow and drastically reduce the impact. In our most recent tests, a single dose of the designed compound reduced the beta-amyloid level by 30 percent." As a therapeutic target, memapsin 2 has an additional advantage because it belongs to a class of enzymes called aspartyl proteases. Researchers already have successfully created drugs to block proteases for the treatment of other diseases. One of these successful drugs was developed from a molecule designed by Ghosh for treatment of drug-resistant HIV, which was approved by the Food and Drug Administration last year. The principles used in the development of these drugs can be carried over and used in the design of new drugs, Tang said. Ghosh's team achieved a breakthrough in Alzheimer's disease research when they were the first to use a method called X-ray crystallography to map the structure of Ghosh's designed inhibitor bound to the enzyme. This revealed information necessary to move the research forward and develop molecules that could be used in drugs. "The moment we had the crystal structure, we knew exactly how the inhibitor worked, the interactions of the molecular bonds and what properties were most important," Ghosh said. "This allowed us to quickly build inhibitor molecules and bypass the usual lengthy process of trial and error in molecule design. Within a year we had developed modified inhibitors that were much smaller and more druglike in character." Ghosh's most recent research findings and the collaborative research results with Tang will be published in the May 3 issue of the Journal of Medicinal Chemistry and are posted on the journal's Web site. Purdue postdoctoral fellow Nagaswamy Kumaragurubaran and graduate students Sarang S. Kulkarni and Xiaoming Xu co-authored the paper. In addition, Lin Hong, Wanpin Chang, Vajira Weerasena, Robert Turner, Gerald Koelsch and Geoffrey Bilcer from the Oklahoma Medical Research Foundation and Athenagen Inc. co-authored the paper. The National Institutes of Health National Institute on Aging funded this research. "We began this work in 2000 and prepared and examined several hundred molecules, we now have one with great clinical potential," Ghosh said. Research into memapsin 2 faced a setback when memapsin 1, an enzyme very similar in structure, was discovered. Unlike memapsin 2, memapsin1 is involved in many important biological processes and its inhibition would cause serious adverse side effects, Ghosh said. "Unfortunately, all of our early designed compounds that were potent against memapsin 2 also inhibited memapsin 1," he said. "Selective inhibition of memapsin 2, or building selectivity, became very important. The scientific community was faced with a formidable challenge." Ghosh's team developed a novel structure-based design strategy to systematically understand where and how to target memapsin 2 specifically. "According to our studies, inhibition of memapsin 2 does not cause toxic side effects," Tang said. "This is extremely encouraging because it allows for intervention very early in the stages of the disease, and it is a type of enzyme with which we are very familiar. There is a precedence of great success in this type of work." Ghosh and Tang founded the biopharmaceutical company Zapaq, located in Oklahoma City, which now has merged with CoMentis. San Francisco-based CoMentis has used the research results of Ghosh and Tang to begin to develop pharmaceuticals. A drug from the memapsin 2 inhibitor could go into the first phase of clinical trials this year and begin the lengthy trial process necessary before the FDA approves a drug to be available on the market. Alzheimer's disease usually begins after age 60, and the risk increases with age. According to the National Institute on Aging, about 5 percent of men and women ages 65-74 have Alzheimer's disease, and nearly half of those 85 and older may have the disease. Study posits new Alzheimer's hypothesis TROY, N.Y., May 30, 2007 (UPI) -- U.S. medical researchers offered a new hypothesis that might be a key to preventing the onset of Alzheimer's disease. Rensselaer Polytechnic Institute scientists are challenging current thinking on the causes and prevention of the disease, finding that a specific imbalance between two peptides might be the cause of the fatal neurological disease that affects more than 5 million people in the United States. "We have found that two peptides, AB42 and AB40, must be in balance for normal function," said Assistant Professor Chunyu Wang, the study's lead researcher. "They are like the Yin and Yang in Taiji, an ancient Chinese philosophy. When the peptides are produced in the correct proportions, the brain is healthy; but when that delicate balance is changed, pathological changes will occur in the brain and the person's memories become hazy, leading to eventual dementia." If Wang's hypothesis is correct, the addition of AB40 might stop the disease's development. Wang notes further research is needed, but his preliminary results challenge the current mode of thinking about how these peptides contribute to the progression of the disease. The research appears in the June edition of the Journal of Molecular Biology.
Aluminum Found In Sunscreen: Could It Cause Skin Cancer? Scientists at Keele University in Staffordshire have questioned the safety of aluminium added to sunscreens and sunblocks. The researchers, Scott Nicholson, BSc, and Dr Christopher Exley, PhD, Birchall Centre for Inorganic Chemistry and Materials Science at Keele, measured the aluminium content of sunscreens/sunblocks, which either include or do not include an aluminium salt (for example, aluminium hydroxide, aluminium oxide, aluminium silicate, aluminium stearate, aluminium starch octenylsuccinate) as an ingredient. Aluminium was present in all seven products tested and its content was of particular significance in three products, each of which listed it as an ingredient. Following numerous enquiries the manufacturers were not forthcoming as to the role of aluminium in their product, except one manufacturer, who confirmed that aluminium hydroxide was added to their product to coat the surface and thereby prevent the agglomeration of another ingredient, titanium dioxide particles. World Health Organisation guidelines recommend a single application of at least 35mL of a sunscreen/sunblock to achieve the stated sun protection factor. For three of the sunscreens/sunblocks investigated a single application of product would result in 200 mg of aluminium being applied to the skin surface. In addition, WHO guidelines suggest re-application of product every two hours which, for example, for an average day on the beach, would result in up to 1g of aluminium being applied to the skin surface. Skin is permeable to aluminium salts when, for example, they are topically applied as antiperspirant formulations. It will accumulate in the skin and be transported to sites throughout the body. It is highly likely that the everyday use of sunscreens/sunblocks is an hitherto unrecognised contributor of aluminium to the human body burden of this non-essential metal. Perhaps of immediate significance is the potential for aluminium in the skin to act as a pro-oxidant. Recent research in the journal Free Radical Biology and Medicine has shown that UV filters in sunscreens promote the formation of reactive oxygen species (ROS) in the nucleated epidermis of the skin. The authors speculate upon the role which might be played by anti-oxidants, either already in the skin or included in sunscreen formulations, in counteracting the pro-oxidant activities of UV filters though they did not consider how the presence of additional pro-oxidants might exacerbate such effects. Aluminium is one such pro-oxidant and could significantly increase the potential for oxidative damage in the skin. While the relationship between the burgeoning use of sunscreens/sunblocks and the increased incidence of skin cancers and, in particular, melanoma, is highly controversial it has not hitherto been considered that aluminium in these products could be an extremely significant contributing factor. Of course, aluminium is already in the skin surface and may not need to be a component of sunscreens/sunblocks to exacerbate oxidative damage attributed to the application of such products. NEW DRUG can reverse the symptoms of Alzheimer's "in minutes"
Comments:
|

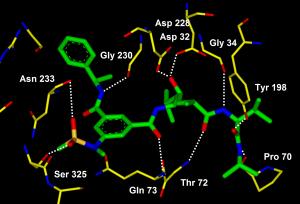 Tang discovered a key enzyme called memapsin 2, or beta-secretase, that is involved in the development of Alzheimer's disease. The action of this enzyme on a special protein, called the amyloid precursor protein, leads to the formation of plaques in the brain. The development of an inhibitor compound targeting memapsin 2 could block this reaction, thus preventing the disease. Utilizing Tang's information about the enzyme, Ghosh designed the first memapsin 2 inhibitor.
Tang discovered a key enzyme called memapsin 2, or beta-secretase, that is involved in the development of Alzheimer's disease. The action of this enzyme on a special protein, called the amyloid precursor protein, leads to the formation of plaques in the brain. The development of an inhibitor compound targeting memapsin 2 could block this reaction, thus preventing the disease. Utilizing Tang's information about the enzyme, Ghosh designed the first memapsin 2 inhibitor.